Chemical Equations and Stoichiometry
Chemical Equations and Stoichiometry: Overview
This topic covers concepts such as Limiting Reagent, Calculation of Number of Moles Using a Limiting Reagent, Calculation of Percentage Yield of a Product Using a Limiting Reagent, Principle of Atomic Conservation, etc.
Important Questions on Chemical Equations and Stoichiometry
In the Haber process of dihydrogen and of dinitrogen were taken for reaction which yielded only of the expected product. What will be the composition of gaseous mixture under the aforesaid condition in the end?
In Haber's process, of dihydrogen and of dinitrogen were taken for the reaction, which yielded only of the expected product. What will be the composition of the gaseous mixture under the aforesaid condition in the end?
If of oxygen combine with to form , the mass of in (Atomic mass of ) used in the reaction is
In an experiment, pure carbon monoxide was passed over red-hot copper oxide. so produced, weighed and the weight of copper oxide was reduced by . Calculate the atomic weight of carbon. [ Take molar mass of carbon dioxide = ]
Equal weights of (atomic weight) and (atomic weight) react to form the compound, . If that is the case, then
If of oxygen combine with to form , the mass of in (Atomic mass of ) used in the reaction is
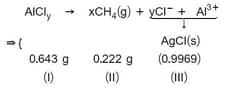
In a chemical determination of the atomic weight of vanadium, of pure was allowed to undergo a set of reactions as a result of which all the chlorine contained in this compound was converted to which weighed . Calculate the atomic weight of vanadium.
The law of constant proportion states that a sample of pure water will always form mass of and mass of irrespective of its source. Find .
The reactant which is entirely consumed in the reaction is known as limiting reagent. In the reaction,
when 5 moles of A react with 6 moles of B then, calculate the amount of C formed.
In the reaction, what will be the number of moles of product formed, starting from mole of , mole of and mole of ?
Round off your answer to the nearest integer after multiplying with 100.
Based on following information determine the value of
The sum of the moles of and will be produced by the combustion of of phosphorous (atomic mass ) in of oxygen, leaving no is . Find the value of (nearest integer).
Chlorophyll, the green colouring matter of plants responsible for photosynthesis, contains of magnesium by mass. Calculate the number of magnesium atoms in of chlorophyll.
Calculate the weight of produced from and .
Given:
If of and of reacts to form water, what is left at the end of the reaction
In an estimation method, of an organic compound gave of nitrogen at STP. Assuming that all the nitrogen in the organic compound is converted to nitrogen gas, the percentage of nitrogen in the organic compound is:
Upon mixing 50.0 mL of 0.1 M lead nitrate solution with 50 mL of 0.05 M chromic sulphate solution, precipitation of lead sulphate solution takes place. How many moles of lead sulphate are formed and what is the molar concentration of chromic sulphate left in the solution?
Which of the following equation does not obey the law of conservation of mass?
In the reaction what will be the number moles of product formed, starting from one mole of moles of and moles of
moles of and of are mixed and allowed to react according to the equation :
What is the maximum number of moles of which could be prepared ?